





Prof. Dr. Katharina Schindowski Zimmermann worked more than 6 years in R&D in Pharma industry (Sanofi, Boehringer Ingelheim) and clinical research (Quintiles) and has successfully developed several AD in vivo models and in vitro assays for compound HTS screening, PK-PD studies, proof-of-concept and mechanism-of action, CNS drug delivery and basic research. She has more than 18 years experience in CNS research and development of therapeutic antibodies with a strong focus on AD. In 2010 she was appointed as full professor for Molecular Pharmacology at Biberach University of Applied Science.
Expertise/Techniques
The World Health Organisation (WHO) estimates that more than a billion people worldwide are suffering from diseases of the CNS. AD is the most common neurodegenerative dementia in the industrialised world, with prevalence rates well over 30% in the over 80-years-old population. AD causes enormous costs to the social healthcare systems, as well as personal tragedies for the patients, families and caregivers. Like most neuro-degenerative diseases AD has a poor prognosis and only symptomatic therapy is currently available. Efficient treatment strategies are still limited and an ageing society in demographic change presents an enormous chal-lenge to the health systems of industrialised nations.
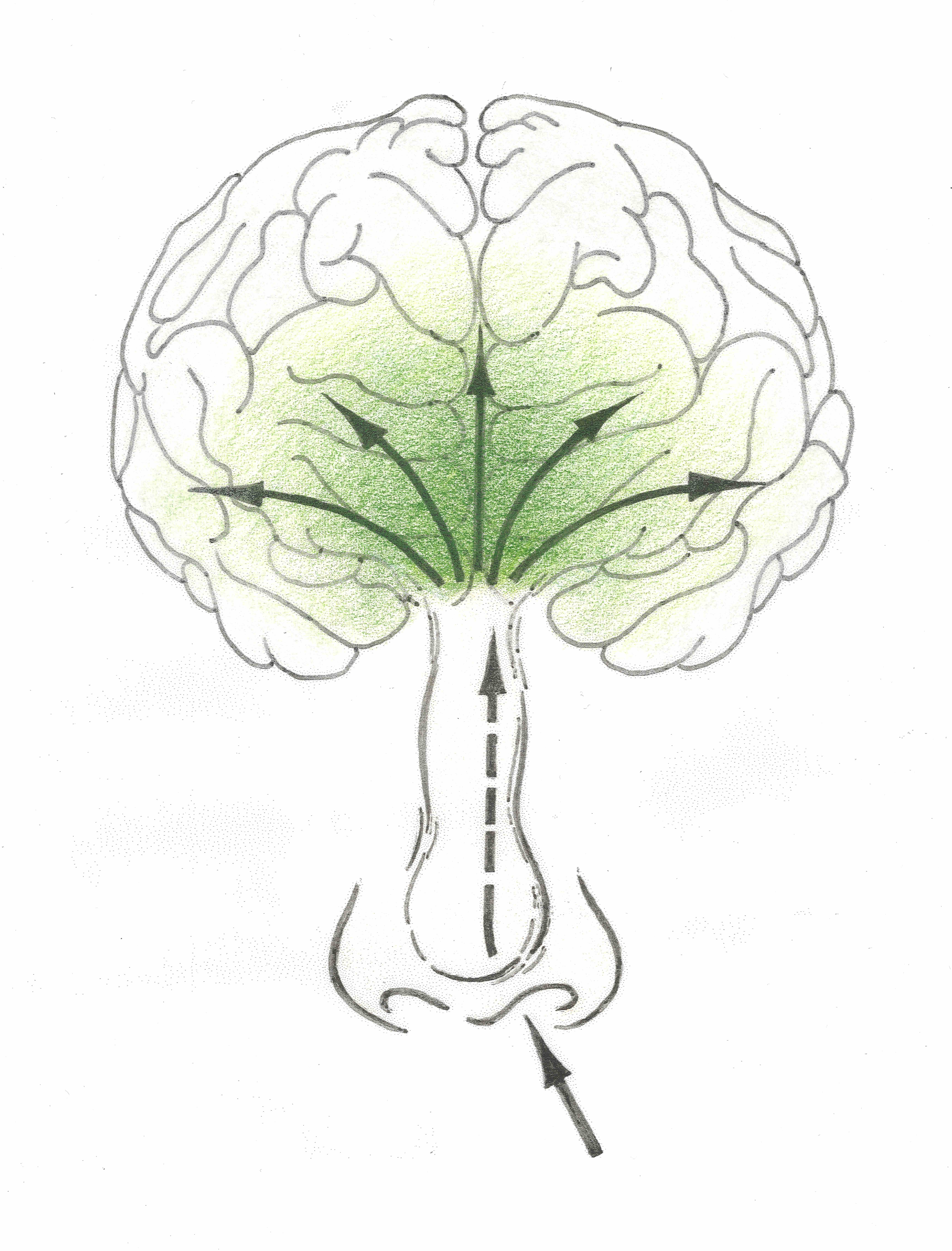
A highly critical point in that context is the low central availability of drugs due to the blood brain barrier (BBB) and nearly all of the larger molecules such as peptides and proteins fail to cross the BBB. The current state-of-the-art to deliver drugs with a low central bioavailability is intrathecal, intracerebroventricular or intraparenchymal injections. They are used to deliver drugs directly to the CSF of the CNS, some of them being given chronically via an implanted intrathecal micropump. Although, such delivery systems are well established, these routes of administration are invasive, require a surgery with high risks, have a lower patient compliance and can be poorly controlled. Intranasal nose to brain (N2B) delivery to the upper third of the nasal cavity bypasses the BBB to rapidly target therapeutics to the CNS along the olfactory and trigeminal neural pathways.
Further reading
Institute member
PhD students
Martin, J.; Rittersberger, R.; Treitler, S.; Kopp, P.; Ibraimi, A.; Koslowski, G.; Sickinger, M.; Dabbars, A.; Schindowski, K. Characterization of a Primary Cellular Airway Model for Inhalative Drug Delivery in Comparison with the Established Permanent Cell Lines CaLu3 and RPMI 2650. In vitro models 2024, 3 (4–6), 183–203. https://doi.org/10.1007/s44164-024-00079-y.
Corazza, E.; Martin, J.; Giordani, B.; Vitali, B.; Rossi, M.; Abruzzo, A.; Bigucci, F.; Cerchiara, T.; di Cagno, M. P.; Luppi, B.; Schindowski, K. Lactobacilli Cell-Free Supernatants: Potential Green and Natural Enhancers for Nose-to-Brain Delivery of Small Hydrophilic Molecules. Journal of Drug Delivery Science and Technology 2024, 99, 105929. https://doi.org/10.1016/j.jddst.2024.105929.
Schindowski, K. Differential Regulation of Neurotrophic Factors During Pathogenic Tau-Aggregation in a Tau Transgenic Mouse Model for Alzheimer’s Disease: A Protocol for Double-Labeling mRNA by In Situ Hybridization and Protein Epitopes by Immunohistochemistry. In Tau Protein; Springer US, 2024; pp 361–385. https://doi.org/10.1007/978-1-0716-3629-9_20.
Ladel, S.; Schindowski, K. Cell-Based in Vitro Models for Nasal Permeability Studies. In Concepts and Models for Drug Permeability Studies; Elsevier, 2024; pp 109–135. https://doi.org/10.1016/b978-0-443-15510-9.00012-8.
Herzog, H.; Glöckler, S.; Flamm, J.; Ladel, S.; Maigler, F.; Pitzer, C.; Schindowski, K. Intranasal Nose-to-Brain Drug Delivery via the Olfactory Region in Mice: Two In-Depth Protocols for Region-Specific Intranasal Application of Antibodies and for Expression Analysis of Fc Receptors via In Situ Hybridization in the Nasal Mucosa. In Tau Protein; Springer US, 2024; pp 387–410. https://doi.org/10.1007/978-1-0716-3629-9_21.
Abdeldaim, D. T.; Schindowski, K. Fc-Engineered Therapeutic Antibodies: Recent Advances and Future Directions. Pharmaceutics2023, 15 (10), 2402. https://doi.org/10.3390/pharmaceutics15102402.
Pernet, V.; Joly, S.; Spiegel, S.; Meli, I.; Idriss, S.; Maigler, F.; Mdzomba, J. B.; Roenneke, A. K.; Franceschini, A.; Silvestri, L.; Pavone, F. S.; Calamai, M.; Schindowski, K.; Chan, A. Nogo-A Antibody Delivery through the Olfactory Mucosa Mitigates Experimental Autoimmune Encephalomyelitis in the Mouse CNS. Cell Death Discovery 2023, 9 (1). https://doi.org/10.1038/s41420-023-01588-7.
Molnar, D.; Röhm, M.; Wutz, J.; Rivec, I.; Michel, A.; Klotz, G.; Hubbuch, J.; Schindowski, K.; Presser, I. A Novel MATLAB®-Algorithm-Based Video Analysis to Quantitatively Determine Solution Creeping in Intact Pharmaceutical Glass Vials. European Journal of Pharmaceutics and Biopharmaceutics 2022, 178, 117–130. https://doi.org/10.1016/j.ejpb.2022.08.003.
Spindler, L. M.; Serpetsi, S.; Flamm, J.; Feuerhake, A.; Böhler, L.; Pravda, M.; Borchers, K.; Tovar, G. E. M.; Schindowski, K.; Gruber-Traub, C. Hyaluronate Spreading Validates Mucin-Agarose Analogs as Test Systems to Replace Porcine Nasal Mucosa Explants – an Experimental and Theoretical Investigation. Colloids and Surfaces B: Biointerfaces 2022, 112689. https://doi.org/10.1016/j.colsurfb.2022.112689.
Fortuna, A.; Schindowski, K.; Sonvico, F. Editorial: Intranasal Drug Delivery: Challenges and Opportunities. Frontiers in Pharmacology 2022, 13. https://doi.org/10.3389/fphar.2022.868986.
Flamm, J.; Hartung, S.; Gänger, S.; Maigler, F.; Pitzer, C.; Schindowski, K. Establishment of an Olfactory Region-Specific Intranasal Delivery Technique in Mice to Target the Central Nervous System. Frontiers in Pharmacology 2022, 12. https://doi.org/10.3389/fphar.2021.789780.
Soleimanizadeh, A.; Dinter, H.; Schindowski, K. Central Nervous System Delivery of Antibodies and Their Single-Domain Antibodies and Variable Fragment Derivatives with Focus on Intranasal Nose to Brain Administration. Antibodies 2021, 10 (4), 47. https://doi.org/10.3390/antib10040047.
Maigler, F.; Ladel, S.; Flamm, J.; Gänger, S.; Kurpiers, B.; Kiderlen, S.; Völk, R.; Hamp, C.; Hartung, S.; Spiegel, S.; Soleimanizadeh, A.; Eberle, K.; Hermann, R.; Krainer, L.; Pitzer, C.; Schindowski, K. Selective CNS Targeting and Distribution with a Refined Region-Specific Intranasal Delivery Technique via the Olfactory Mucosa. Pharmaceutics 2021, 13 (11), 1904. https://doi.org/10.3390/pharmaceutics13111904.
Spindler, L. M.; Feuerhake, A.; Ladel, S.; Günday, C.; Flamm, J.; Günday-Türeli, N.; Türeli, E.; Tovar, G. E. M.; Schindowski, K.; Gruber-Traub, C. Nano-in-Micro-Particles Consisting of PLGA Nanoparticles Embedded in Chitosan Microparticles via Spray-Drying Enhances Their Uptake in the Olfactory Mucosa. 2021, 12. https://doi.org/10.3389/fphar.2021.732954.
Ladel, S.; Maigler, F.; Flamm, J.; Schlossbauer, P.; Handl, A.; Hermann, R.; Herzog, H.; Hummel, T.; Mizaikoff, B.; Schindowski, K. Impact of Glycosylation and Species Origin on the Uptake and Permeation of IgGs through the Nasal Airway Mucosa. 2020, 12 (11), 1014. https://doi.org/10.3390/pharmaceutics12111014.
Dinter, H.; Zadeh, A. S.; Zimmermann, K. S. In Vitro-Generation von Antikörpern Mittels Phagen-Display. BIOspektrum 2020, 26 (3), 269–272. https://doi.org/10.1007/s12268-020-1371-9.
Ladel, S.; Schlossbauer, P.; Flamm, J.; Luksch, H.; Mizaikoff, B.; Schindowski, K. Improved In Vitro Model for Intranasal Mucosal Drug Delivery: Primary Olfactory and Respiratory Epithelial Cells Compared with the Permanent Nasal Cell Line RPMI 2650. Pharmaceutics2019, 11 (8), 367. https://doi.org/10.3390/pharmaceutics11080367.
Solemani Zadeh, A.; Grässer, A.; Dinter, H.; Hermes, M.; Schindowski, K. Efficient Construction and Effective Screening of Synthetic Domain Antibody Libraries. Methods and Protocols 2019, 2 (1), 17. https://doi.org/10.3390/mps2010017.
Gänger, S.; Schindowski, K. Tailoring Formulations for Intranasal Nose-to-Brain Delivery: A Review on Architecture, Physico-Chemical Characteristics and Mucociliary Clearance of the Nasal Olfactory Mucosa. Pharmaceutics 2018, 10 (3), 116. https://doi.org/10.3390/pharmaceutics10030116.
Ladel, S.; Flamm, J.; Zadeh, A. S.; Filzwieser, D.; Walter, J.-C.; Schlossbauer, P.; Kinscherf, R.; Lischka, K.; Luksch, H.; Schindowski, K. Allogenic Fc Domain-Facilitated Uptake of IgG in Nasal Lamina Propria: Friend or Foe for Intranasal CNS Delivery? Pharmaceutics 2018, 10 (3), 107. https://doi.org/10.3390/pharmaceutics10030107.
Schindowski-Zimmermann, K. Regulation of Neurotrophic Factors during Pathogenic Tau-Aggregation: A Detailed Protocol for Double-Labeling mRNA by in Situ Hybridization and Protein Epitopes by Immunohistochemistry. Methods in Molecular Biology 2017, 1523, 391–414.
Röhm, M.; Carle, S.; Maigler, F.; Flamm, J.; Kramer, V.; Mavoungou, C.; Schmid, O.; Schindowski, K. A Comprehensive Screening Platform for Aerosolizable Protein Formulations for Intranasal and Pulmonary Drug Delivery. International Journal of Pharmaceutics 2017, 532 (1), 537–546.
Röhm, M.; Handl, A.; König, M.; Mavoungou, C.; Handrick, R.; Schindowski, K. Data of Rational Process Optimization for the Production of a Full IgG and Its Fab Fragment from Hybridoma Cells. Data in Brief 2016, 8, 426–435.
Engelhardt, L.; Röhm, M.; Mavoungou, C.; Schindowski, K.; Schafmeister, A.; Simon, U. First Steps to Develop and Validate a CFPD Model in Order to Support the Design of Nose-to-Brain Delivered Biopharmaceuticals. Pharmaceutical Research 2016, 33 (6), 1337–1350.
Stützle, M.; Flamm, J.; Carle, S.; Schindowski, K. Nose-to-Brain Delivery of Insulin for Alzheimer’s Disease. ADMET and DMPK 2015, 3 (3), 190–202.
Moreth, J.; Mavoungou, C.; Schindowski, K. Passive Anti-Amyloid Immunotherapy in Alzheimer’s Disease: What Are the Most Promising Targets? Immunity and Ageing 2013, 10 (1).
Moreth, J.; Mavoungou, C.; Schindowski, K. Is Abeta a Sufficient Biomarker for Monitoring Anti-Abeta Clinical Studies? A Critical Review. Frontiers in Aging Neuroscience 2013, 5 (JUL).
Schindowski, K.; Von Bohlen Und Halbach, O.; Strelau, J.; Ridder, D. A.; Herrmann, O.; Schober, A.; Schwaninger, M.; Unsicker, K. Regulation of GDF-15, a Distant TGF-? Superfamily Member, in a Mouse Model of Cerebral Ischemia. Cell and Tissue Research 2011, 343 (2), 399–409.
Belarbi, K.; Burnouf, S.; Fernandez-Gomez, F.; Desmercières, J.; Troquier, L.; Brouillette, J.; Tsambou, L.; Grosjean, M.; Caillierez, R.; Demeyer, D.; Hamdane, M.; Schindowski, K.; Blum, D.; Buée, L. Loss of Medial Septum Cholinergic Neurons in THY-Tau22 Mouse Model: What Links with TAU Pathology? Current Alzheimer Research 2011, 8 (6), 633–638.
Belarbi, K.; Schindowski, K.; Burnouf, S.; Caillierez, R.; Grosjean, M.-E.; Demeyer, D.; Hamdane, M.; Sergeant, N.; Blum, D.; Buée, L. Early Tau Pathology Involving the Septo-Hippocampal Pathway in a Tau Transgenic Model: Relevance to Alzheimer’s Disease. Current Alzheimer Research 2009, 6 (2), 152–157.
Schindowski, K.; Belarbi, K.; Buée, L. Neurotrophic Factors in Alzheimer’s Disease: Role of Axonal Transport. Genes, Brain and Behavior 2008, 7 (SUPPL. 1), 43–56.
Schindowski, K.; Belarbi, K.; Bretteville, A.; Ando, K.; Buée, L. Neurogenesis and Cell Cycle-Reactivated Neuronal Death during Pathogenic Tau Aggregation. Genes, Brain and Behavior 2008, 7 (SUPPL. 1), 92–100.
Krause, S.; Schindowski, K.; Zechel, S.; Von Bohlen Und Halbach, O. Expression of trkB and trkC Receptors and Their Ligands Brain-Derived Neurotrophic Factor and Neurotrophin-3 in the Murine Amygdala. Journal of Neuroscience Research 2008, 86 (2), 411–421.
Schindowski, K.; Eckert, A.; Peters, J.; Gorriz, C.; Schramm, U.; Weinandi, T.; Maurer, K.; Frölich, L.; Müller, W. E. Increased T-Cell Reactivity and Elevated Levels of CD8+Memory T-Cells in Alzheimer’s Disease-Patients and T-Cell Hyporeactivity in an Alzheimer’s Disease-Mouse Model: Implications for Immunotherapy. NeuroMolecular Medicine 2007, 9 (4), 340–354.
Leuner, K.; Pantel, J.; Frey, C.; Schindowski, K.; Schulz, K.; Wegat, T.; Maurer, K.; Eckert, A.; Müller, W. E. Enhanced Apoptosis, Oxidative Stress and Mitochondrial Dysfunction in Lymphocytes as Potential Biomarkers for Alzheimer’s Disease. Journal of Neural Transmission, Supplementa 2007, No. 72, 207–215.
Leroy, K.; Bretteville, A.; Schindowski, K.; Gilissen, E.; Authelet, M.; De Decker, R.; Yilmaz, Z.; Buée, L.; Brion, J.-P. Early Axonopathy Preceding Neurofibrillary Tangles in Mutant Tau Transgenic Mice. American Journal of Pathology 2007, 171 (3), 976–992.
Schindowski, K.; Peters, J.; Gorriz, C.; Schramm, U.; Weinandi, T.; Leutner, S.; Maurer, K.; Frölich, L.; Müller, W. E.; Eckert, A. Apoptosis of CD4+ T and Natural Killer Cells in Alzheimer’s Disease. Pharmacopsychiatry 2006, 39 (6), 220–228.
Schindowski, K.; Bretteville, A.; Leroy, K.; Bégard, S.; Brion, J.-P.; Hamdane, M.; Buée, L. Alzheimer’s Disease-like Tau Neuropathology Leads to Memory Deficits and Loss of Functional Synapses in a Novel Mutated Tau Transgenic Mouse without Any Motor Deficits. American Journal of Pathology 2006, 169 (2), 599–616.
Leutner, S.; Schindowski, K.; Frölich, L.; Maurer, K.; Kratzsch, T.; Eckert, A.; Müller, W. E. Enhanced ROS-Generation in Lymphocytes from Alzheimer’s Patients. Pharmacopsychiatry 2005, 38 (6), 312–315.
Hamdane, M.; Bretteville, A.; Sambo, A.-V.; Schindowski, K.; Bégard, S.; Delacourte, A.; Bertrand, P.; Buée, L. P25/Cdk5-Mediated Retinoblastoma Phosphorylation Is an Early Event in Neuronal Cell Death. Journal of Cell Science 2005, 118 (6), 1291–1298.
Kiprianova, I.; Schindowski, K.; Von Bohlen Und Halbach, O.; Krause, S.; Dono, R.; Schwaninger, M.; Unsicker, K. Enlarged Infarct Volume and Loss of BDNF mRNA Induction Following Brain Ischemia in Mice Lacking FGF-2. Experimental Neurology 2004, 189 (2), 252–260.
Schindowski, K.; Kratzsch, T.; Peters, J.; Steiner, B.; Leutner, S.; Touchet, N.; Maurer, K.; Czech, C.; Pradier, L.; Frölich, L.; Müller, W. E.; Eckert, A. Impact of Aging: Sporadic, and Genetic Risk Factors on Vulnerability to Apoptosis in Alzheimer’s Disease. NeuroMolecular Medicine 2003, 4 (3), 161–177.
Eckert, A.; Keil, U.; Kressmann, S.; Schindowski, K.; Leutner, S.; Leutz, S.; Müller, W. E. Effects of EGb 761? Ginkgo Biloba Extract on Mitochondrial Function and Oxidative Stress. Pharmacopsychiatry2003, 36 (SUPPL. 1).
Schindowski, K.; Fröhlich, L.; Maurer, K.; Müller, W. E.; Eckert, A. Age-Related Impairment of Human T Lymphocytes’ Activation: Specific Differences between CD4+and CD8+Subsets. Mechanisms of Ageing and Development 2002, 123 (4), 375–390.
Schindowski, K.; Leutner, S.; Kressmann, S.; Eckert, A.; Müller, W. E. Age-Related Increase of Oxidative Stress-Induced Apoptosis in Mice Prevention by Ginkgo Biloba Extract (EGb761). Journal of neural transmission (Vienna, Austria : 1996) 2001, 108 (8–9), 969–978.
Schindowski, K.; Leutner, S.; Kressmann, S.; Eckert, A.; Müller, W. E. Age-Related Increase of Oxidative Stress-Induced Apoptosis in Mice Prevention by Ginkgo Biloba Extract (EGb761). Journal of Neurology 2001, 248 (9), 969–978.
Eckert, A.; Schindowski, K.; Leutner, S.; Luckhaus, C.; Touchet, N.; Czech, C.; Müller, W. E. Alzheimer’s Disease-like Alterations in Peripheral Cells from Presenilin-1 Transgenic Mice. Neurobiology of Disease 2001, 8 (2), 331–342.
Schindowski, K.; Leutner, S.; Müller, W. E.; Eckert, A. Age-Related Changes of Apoptotic Cell Death in Human Lymphocytes. Neurobiology of Aging 2000, 21 (5), 661–670.
Leutner, S.; Czech, C.; Schindowski, K.; Touchet, N.; Eckert, A.; Müller, W. E. Reduced Antioxidant Enzyme Activity in Brains of Mice Transgenic for Human Presenilin-1 with Single or Multiple Mutations. Neuroscience Letters 2000, 292 (2), 87–90.
Pitzer, C.; Schindowski, K.; Pomer, S.; Wirth, T.; Zöller, M. In Vivo Manipulation of Interleukin-2 Expression by a Retroviral Tetracycline (Tet)-Regulated System. Cancer Gene Therapy 1999, 6 (2), 139–146.
